November 15th, 2025
UPL (Upper Payment Limit) Supplemental Payment Program FFY 2026 Eligibility List Published
The HHSC Provider Finance Department has published the eligibility list for the federal fiscal year (FFY) 2026 Intermediate Care Facilities for Individuals with an Intellectual Disability or Related Conditions (ICF/IID) Upper Payment Limit (UPL) supplemental payment program on the program webpage.
Per Title 1 of the Texas Administrative Code, Section 355.458, non-state government-owned ICF/IID providers are eligible for this supplemental payment program.
Under this program, a participating non-state government entity would contribute the “state share” of the supplemental payment in the form of inter-governmental transfers (IGTs). The state will claim matching funds and will make a supplemental payment to the ICF/IID facility based on the supplemental amount calculated by the state and the IGT amount transferred by the entity.
Enrollment for the FFY 2026 program will open on Dec. 1, 2025, and will last for 30 days.
To increase transparency with the program we are sharing additional information to include a list of facilities we deemed eligible to participate in the voluntary program based on the eligibility criteria. This includes the eligibility list, which reflects which facilities are not eligible and which facilities are eligible. If a facility has a question related to our initial eligibility assessment, they can contact Provider Finance. “
FFY 2026 ICF UPL Supplemental Payment Program Eligibility List (.xlsx)
ICF/IID UPL Supplemental Payment Program FFY 2026 Eligibility List Published
The HHSC Provider Finance Department has published the eligibility list for the federal fiscal year (FFY) 2026 Intermediate Care Facilities for Individuals with an Intellectual Disability or Related Conditions (ICF/IID) Upper Payment Limit (UPL) supplemental payment program on the program webpage.
Per Title 1 of the Texas Administrative Code, Section 355.458, non-state government-owned ICF/IID providers are eligible for this supplemental payment program. Under this program, a participating non-state government entity would contribute the “state share” of the supplemental payment in the form of inter-governmental transfers (IGTs). The state will claim matching funds and will make a supplemental payment to the ICF/IID facility based on the supplemental amount calculated by the state and the IGT amount transferred by the entity.
Enrollment for the FFY 2026 program will open on Dec. 1, 2025, and will last for 30 days. HHSC will publish both a link to the enrollment portal and a GovDelivery alert when the enrollment period opens.
**HHSC will publish both a link to the enrollment portal and a GovDelivery alert when the enrollment period opens.
September 15th, 2025
Updated ICF/IID Services Payment Rates
HHSC has published updated fee schedules for Intermediate Care Facilities for Individuals with an Intellectual Disability or Related Conditions (ICF/IID) regarding the approved payment rates for services.
The HHSC Provider Finance Department (PFD) webpage has more information about the approved payment rates.
If you have further questions, email HHSC PFD, Long-term Services and Supports (LTSS), Center for Information and Training, or call us at (737) 867-7817.
June 26th, 2025
HHSC Publishes Revised Process for Reporting Incidents, Complaints and HHSC Investigations of Abuse, Neglect, and Exploitation (PL 2025-02) for ICF
HHSC Long-Term Care Regulation updated Provider Letter (PL) 2025-02 Process for Reporting Incidents, Complaints and HHSC Investigations of Abuse, Neglect, and Exploitation for Intermediate Care Facilities for Individuals with an Intellectual Disability (ICF/IID) to include the facility must report the alleged abuse, neglect, and exploitation (ANE) to HHSC Complaint and Incident Intake within one hour of suspecting or learning of the allegation. This includes instances in which the facility learns of the alleged ANE when the surveyor enters the ICF on the complaint investigation and informs the provider of the allegation. This PL replaces PL 2024-04.
For questions contact LTCRPolicy@hhs.texas.gov.
April 8th, 2025
April & May Trainings for ICF
Reporting Abuse, Neglect, Exploitation Changes and Self-Reporting Incidents for Intermediate Care Facilities
Wednesday, April 9
10 – 11 a.m.
Register here for the webinar.
Reporting Abuse, Neglect, Exploitation Changes and Self-Reporting Incidents for Intermediate Care Facilities
Thursday, May 22
2 – 3 p.m.
Register here for the webinar.
Hurricane Season – Ready or Not! Preparedness for Intermediate Care Facilities (ICF)
Wednesday, May 28
10 a.m. – noon
Register here for the webinar.
February 22nd, 2025
HHSC LTCR Updates Tag Crosswalks for Intermediate Care Facilities
LTCR has updated the ICF federal, licensure and state standards of participation tag crosswalks to the most current version.
Each crosswalk provides a detailed table that identifies the applicable tag numbers, title, Code of Federal Regulations and Texas Administrative Codes for providers to reference.
These documents are posted on the ICF Provider Portal HHSC website.
For questions, email LTCRPolicy@hhs.texas.gov.
November 13th, 2024
HHSC Rescinds PL 2024-20 Requirements for Tuberculosis Screening and Testing
HHSC rescinded PL 2024-20 Requirements for Tuberculosis Screening and Testing. Additional guidance regarding tuberculosis screening and testing will be forthcoming.
In the interim, providers should follow their program regulations and internal communicable disease and infection control policies and procedures to address tuberculosis screening and testing.
Providers may use recommendations from the Centers for Disease Control and the Texas Department of State Health Services to inform their policies.
For more information, follow the links below:
Frequently Asked Questions About TB | Texas DSHS
Tuberculosis Screening for Adults in Various Settings (PDF)
August 12th, 2024
PL 24-04 –Changes & Guidance To ICF/IID Provider Reporting Processes and HHSC Investigating Processes- Effective March 1, 2024.
As the ICF/IID rules to implement HB 4696 applies to the 6-13 bed facilities and is not yet adopted, yet the provisions in the bills became effective 9/1/2023. We are unclear if or how HHSC is monitoring for compliance as the only guidance released to date is PL 24-04 . And, this PL only addresses the provider reporting processes and HHSC investigating processes which became effective March 1, 2024. The PL does not provide guidance or address compliance related to the directives in the bill which, as of September 1, 2023, is now law.
In response to inquiries from providers and provider associations, HHSC has now stated that “at this time ICF/IID surveyors are surveying for compliance with the current regulations located in 26 TAC 551 and guidance issued in PL 2024-04.“
NOTE: HCS/TxHmL: It’s anticipated the rules to implement HB 1009 in HCS/TxHmL will be posted for ‘informal’ comment this fall. Rules to implement HB 4696 are postponed until after the 89th Session.
March 10th, 2024
ICF/IID ANE Webinar- Very Important!
Thursday, March 21
2–3:30 p.m.
Register here for webinar.
HHSC Long-term Care Regulation has published Provider Letter 2024-04 Revised (Replaces PL 17-02 and 17-03) Revised Process for Reporting and HHSC Investigations of Abuse, Neglect, and Exploitation for Intermediate Care Facilities for Individuals with an Intellectual Disability (ICF/IID). Don’t forget, as of March 1st, 2024, this replaces the previous ANE Reporting & Investigations process. Reporting is now done to (CII) Complaint & Incident Intake.
February 25th, 2024
New Reporting Process for ANE Reporting & Investigations for ICF/IID Program
HHSC has released a new letter of guidance regarding the revised ANE reporting process to CII and the ANE investigation process for ICF/IID. Here is the published provider letter (PL) 2024-04
Beginning March 1, 2024, reporting of investigations for allegations of abuse, neglect, and exploitation (ANE) for Medicaid consumers served by an Intermediate Care Facility for Intellectual and Developmental Disabilities (ICF/IDD) will transition from the Department of Family and Protective Services Statewide Intake (DFPS SWI) to HHSC Regulatory Services Complaint and Incident Intake (CII).
Additionally, processes related to investigations of ANE of ICF/IDD will change as of March 1, 2024.
For questions contact LTCRPolicy@hhs.texas.gov.
January 20th, 2024
Surrogate Decision-Making Program (SDMP)
Randy Rowley, Manager, SDMP, HHSC, provided an overview of the surrogate decision-making program to the workgroup. Click here for link to ppt
HHSC December 27, 2023 Bulletin:
The legislatively mandated Surrogate Decision-making Program (SDMP) authorizes Surrogate Consent Committees (SCCs), comprised of trained volunteers, to provide written informed consent for individuals residing in community-based ICFs/IID who have been assessed to lack the capacity to make certain treatment decisions for themselves and have no legal guardian or actively involved family members serving as Surrogate Decision Makers (SDMs) or the treatment decisions are beyond the scope of the SDMs.
The SDMP facilitates compliance with Code of Federal Regulations (CFR) Title 42 and state ICF/IID rules regarding protection of individuals rights.
For the list of treatment decisions that a SCC can make see the HHS SDMP webpage.
Watch for upcoming training event announcements.
For questions about SCCs, or if you want to volunteer to serve on a SCC, email SDMP Manager Randy Rowley or call 512-438-4306.
ICF Rules-TAC Code Draft Title 26 Part I Ch 551
Personal Needs Allowance Information Letter
Personal Needs Allowance (PNA): PNA of individuals residing in a nursing home or ICF/IID from $60/month to $75/month, and for couples from $120/month to $150/month effective January 1, 2024. HHSC posted an information letter regarding this change:
IL 2023-45 Personal Needs Allowance (PNA) Adjustment (Replaces IL 2022-16) (texas.gov).
June 15th, 2023
Personal Needs Allowance Increase Bill passed during 88th legislature session
December 15th, 2022
ICF Resources
These resources can also be found on the ICF Provider Portal page.
September 25th, 2022
HHSC Publishes Updated Guidance on the Amelioration of Administrative Penalties for ALF, ICF/IID, and NF Providers
HHSC Long-term Care Regulation has published Provider Letter 2022-24 – Amelioration of Administrative Penalties (replaces PL 2013-18).
The letter provides guidelines to assisted living facilities,
intermediate care facilities for individuals with an intellectual
disability or related conditions, and nursing facilities about
the amelioration of administrative penalties assessed for state licensure
violations.
Read the provider letter details.
June 12th, 2022
ICF COVID-19 Vaccination Reporting Emergency Rules Expired June 6!!
Emergency rules for Intermediate Care Facilities for Individuals with an Intellectual Disability or Related Conditions that require COVID-19 vaccination reporting expired June 6.
This means that effective June 7, ICFs no longer have to report COVID-19 vaccination data for staff and individuals to HHSC.
The following rules expired June 6:
- 26 TAC §551.48 – ICF/IID Provider COVID-19 Vaccination Data Reporting Requirement.
Here is a copy of the previous rule that expired:
551.48.ICF/IID Provider COVID-19 Vaccination Data Reporting Requirement.
(a) An intermediate care facility administrator and one additional designee must enroll in an emergency communication system in accordance with instructions from Texas Health and Human Services Commission (HHSC).
(b) An intermediate care facility must respond to requests for information received through the emergency communication system in the format established by HHSC.
(c) Within 24 hours of becoming aware of a staff or resident’s COVID-19 vaccination, an intermediate care facility must accurately report COVID-19 vaccination data for staff and individuals in the format established by HHSC.
(d) Subsection (c) of this section does not apply to state supported living centers.
The agency certifies that legal counsel has reviewed the emergency adoption and found it to be within the state agency’s legal authority to adopt.
Filed with the Office of the Secretary of State on August 10, 2021.
Email questions to LTCR Policy.
April 29th, 2022
ICF Visitation Rules Update
An alert went out to remind providers that all visitation must be allowed. Essential caregiver and end-of-life visits must be allowed for all individuals with any COVID-19 status. A facility may be cited if visitation is not allowed.
Review ICF/IID visitation rules from April 4th, 2022, for additional information.
April 10th, 2022
COVID-19 ICF Mitigation, Response Rule Revised Effective April 6
HHSC Long-term Care Regulation has published a revised Intermediate Care Facilities for Individuals with an Intellectual Disability or Related Conditions COVID-19 Mitigation and Response Emergency Rule. It is effective April 6, 2022.
The revised rule:
- Removes the requirements to have plans for obtaining and maintaining a two-week supply of full PPE.
- Clarifies that a facility does not have to provide the name of the person who tested positive for COVID-19 when reporting to CII.
April 10th, 2022
HHSC Publishes Description of Key Changes to 26 TAC 551, ICF/IID (PL 2022-07)
HHSC has published Provider Letter 2022-07, Description of Key Changes to 26 TAC 551, Intermediate Care Facilities for Individuals with an Intellectual Disability or Related Conditions, for ICF/IID providers. This letter describes the key changes to Title 26 of the Texas Administrative Code, Chapter 551, that were effective on Feb. 24.
Throughout the rule, HHSC updated citations, agency names, and terminology; corrected minor grammatical and punctuation errors; and revised sentence structures to make the chapter easier to read. In addition, the following items are addressed in this letter:
– New Requirements for Infection Prevention and Control Policies and Procedures
-HHSC included a state rule that refers to each Centers for Medicare & Medicaid Services (CMS) Condition of Participation (CoP). Additions to 26 TAC 551 include • Governing Body • Client Protections • Facility Staffing • Active Treatment • Client Behavior and Facility Practices • Health Care Services • Physical Environment • Emergency Preparedness • Dietetic Services.
– transition from paper applications to the use of the online licensure portal, called the Texas Unified Licensure Information Portal (TULIP) (Disclose information when applying for “relocation” and application information must be submitted through portal in “TULIP” system)
-Now require evaluation of the emergency preparedness and response plan at least every two years, instead of annually.
-ANE & Incident definitions
-Administrative penalties for each visit are limited to the cap amount, regardless of the number or duration of violations as of Sept. 1st, 2021
****If you have any questions about this letter, please contact the Policy and Rules Section by email at LTCRPolicy@hhs.texas.gov or call (512) 438-3161
April 6th, 2022
COVID Screening in ICF’s
ICF COVID-19 Mitigation and Provider Response emergency rules require an intermediate care facility must screen individuals according to HHSC guidance
.ICFs must screen individuals:
•upon admission or readmission to the facility; and
•at least once a day.
ICFs must screen each employee or contractor for the following criteria (listed below) before entering the facility at the start of their shift.
•Staff screenings must be documented in a log kept at the facility entrance and must include the name of each person screened, the date and time of the evaluation, and the results of the evaluation.
**Staff who meet any of the criteria must not be permitted to enter the facility.
As per ICF/IID Expansion of Reopening Visitation Emergency rules, ICFs are required to screen all visitors for signs or symptoms of COVID-19.
*Visitor screenings must be documented in a log kept at the entrance to the facility, which must include the name of each person screened, the date and time of the screening, and the results of the screening. The visitor screening log may contain protected health information and must be protected in accordance with applicable state and federal law .
*A visitor may not participate in a visit if the visitor has signs and symptoms of COVID-19 or active COVID-19 infection.
Screening Criteria:
•fever, defined as a temperature of 100.4 Fahrenheit and above, or
signs or symptoms of a respiratory infection, such as cough, shortness of breath, or sore throat;
•other signs or symptoms of COVID-19, including
-chills,
-new or worsening cough,
-shortness of breath or difficulty breathing,
-fatigue,
-muscle or body aches,
-headache,
-new loss of taste or smell,
-sore throat,
-congestion or runny nose,
-nausea or vomiting,
-or diarrhea;
•any other signs and symptoms as outlined by the CDC in Symptoms or Coronavirus at cdc.gov;
•contact in the last 14 days with someone who has a confirmed diagnosis of COVID-19, is under investigation for COVID-19, or is ill with a respiratory illness, regardless of whether the person is fully vaccinated, unless the person is entering the facility to provide critical assistance; or
•testing positive for COVID-19 in the last 10 days.
April 6th, 2022
Reporting Confirmed Case of COVID-19 in ICF/IID
A facility must notify the Texas Health and Human Services Commission (HHSC) Complaint and Incident Intake of COVID-19 activity as described below.
(1) A facility must notify HHSC of the first confirmed case of COVID-19 in staff or individuals, and the first confirmed case of COVID-19 after a facility has been without cases for 14 days or more, at HHSC Complaint and Incident Intake (CII) through TULIP, or by calling 1-800-458-9858, within 24 hours of the positive confirmation.
(2) A facility must submit a Form 3613-A Provider Investigation Report, minus the name of the person who tested positive for COVID-19, to HHSC Complaint and Incident Intake, through TULIP, by email at ciiprovider@hhs.texas.gov, or by fax at 877-438-5827, within five working days from the day a confirmed case is reported to CII.
April 6th, 2022
COVID-19 Mitigation and Response Emergency Rule Updated
HHSC Long-term Care Regulation has published a revised Intermediate Care Facilities for Individuals with an Intellectual Disability or Related Conditions COVID-19 Mitigation and Response Emergency Rule.
It became effective April 6, 2022.
The revised rule:
•Points to guidance from the Texas Department of State Health Services and HHSC rather than the CDC.
•Removes the requirements to have plans for obtaining and maintaining a two-week supply of full PPE.
•Removes the requirement to have spaces to don and doff PPE
•Clarifies that a facility does not have to provide the name of the person who tested positive for COVID-19 when reporting to CII.
February 27th, 2022
HHSC Adopts Revised ICF/IID Rules – Effective Feb. 24th, 2022
HHSC Long-term Care Regulation has adopted updates to the Intermediate Care Facilities for Individuals with an Intellectual Disability (ICF/IID) or Related Conditions program rules. The revised rules are in the Texas Administrative Code Title 26, Chapter 551. They are effective Feb. 24, 2022.
Key changes to the rule are to:
- Implement House Bill 1848 from the 86th Legislature, Regular Session, 2019 which requires new infection control policies and procedures in long-term living facilities.
- Implement House Bill 3720 from the 87th Legislature, Regular Session, 2021 which limits the total amount of an administrative penalty assessed against an ICF/IID.
- Reintegrate the conditions of participation from the Code of Federal Regulations.
- Corrects legacy agency terms, update rule citations, and edit for clarity and consistency.
COVID-19 ICF/IID Webinar Recording from Feb. 14 Available
A recording of the Feb. 14 Intermediate Care Facilities for Individuals with an Intellectual Disability or Related Conditions COVID-19 Q&A with HHSC Long-term Care Regulation and DSHS is available for those who could not attend.
There have been changes made to the PowerPoint based on the information provided by DSHS during the webinar.
Listen to the webinar recording.
Email LTCR Policy for the transcript.
February. 20th, 2022
Mar. 07 ICF COVID-19 Webinar with HHSC LTCR
Long-term Care Regulation and the Department of State Health Services will provide the latest information on the COVID-19 pandemic and take live questions from participants in this intermediate care facilities provider webinar.
Provider attendance is critical to staying current with COVID-19 requirements and guidance. ICF/IID providers are strongly encouraged to attend all COVID-19 webinars with LTCR and DSHS.
Those using Internet Explorer may have difficulties registering for the webinar. If so, try using another browser, such as Google Chrome or Microsoft Edge.
March 7, 2022
11 a.m.–12 p.m.
Register for the COVID-19 Webinar.
February 13th, 2022
HHSC is not currently assessing compliance with CMS’s Omnibus COVID-19 Health Care Staff Vaccination rules, published in the Federal Register on November 5, 2021.
ICF COVID-19 Vaccination Data Reporting and Emergency Communication System – Feb 7
HHSC Long-term Care Regulation has published a revised ICF/IID Provider COVID-19 Vaccination Data Reporting Rule (PDF). It became effective February 7, 2022 and includes Emergency Communication System Enrollment for Intermediate Care Facilities for Individuals with an Intellectual Disability or Related Conditions providers.
The rule requires ICF/IIDs to:
- Report COVID-19 vaccine data within 24 hours
- Enroll in an emergency communication system
January 7th, 2022
ICF COVID-19 Response Plan and FAQ Updated – Jan. 7
Document Version Date Change Comments
Version 3.5 1/5/2022 Changes to pages 6, 31, 38, 39, 40, 51, 72 71, and Changes made to reflect the most updated CDC guidance.
Version 3.4 12/07/2021 Changes to pages 15, 16, 25, 26, 31, 35 Edited to include revised ICF COVID-19 Provider Response Mitigation Rules for ICFs/IID
For changes made previous to 12/07/2021, please read the Table of Changes starting with page 7 of 19 of the Response Plan.
Update your Infection Control Policies and other related policies accordingly.
HHSC has revised the ICF COVID-19 Response Plan and Frequently Asked Questions document in response to the most recent CDC guidance.
November 21st, 2021
HHSC Publishes PL 2021-38 Medicaid Bed Reallocation
HHSC published Provider Letter 2021-38 Medicaid Bed Reallocation which explains the process to request reallocated ICF/IID Medicaid beds from HHSC. This letter replaces Provider Letter 2019-21.
November 4th, 2021
Updated ICF/IID COVID-19 FAQ and COVID-19 Response Plan Revised
HHSC has revised the Frequently Asked Questions for ICF/IIDs about COVID-19 (PDF) and the ICF/IID COVID-19 Response Plan (PDF) in response to the revised COVID-19 Expansion of Reopening Visitation for ICF Providers rules.
October 24th, 2021
New PL 2021-21 COVID-19 – Expansion of Reopening Visitation for ICF Providers
Super important!!!!
HHSC has published Provider Letter 2021-21, COVID-19 Response – Expansion of Reopening Visitation (PDF) for ICF/IID providers. This letter replaces Provider Letter 2021-10. This letter describes the criteria for expanded visitation as well as address changes in response to Executive Order No. GA-38(link is external) and updated CDC guidance.
Updated COVID-19 Expansion of Reopening Visitation Emergency Rules for ICF Providers
HHSC Long-term Care Regulation has published revised COVID-19 Expansion of Reopening Visitation Emergency Rules for Intermediate Care Facilities for Individuals with Intellectual Disabilities (ICFs/IID) or Related Conditions (PDF). The rules address changes in response to Executive Order No. GA-38 (PDF)(link is external) and updated CDC guidance.The rules became effective on October 20, 2021.
September 15th, 2021
HHSC Publishes Amended Statutory Cap Regarding Administrative Penalties for ICFs: (PL 21-34)
HHSC published Provider Letter 21-34 Amended Statutory Cap Regarding Administrative Penalties for Intermediate Care Facilities. PL 21-34 notifies providers of changes to how HHSC determines and imposes administrative penalties based on changes made by House Bill 3720 (87th Legislature, Regular Session, 2021).
September 5th, 2021
Guidance for Providers Regarding Entry into LTC Facilities (PL 2021-33)
HHSC Long-term Care Regulation has published Provider Letter 2021-33, Authority to Enter Long-term Care Facilities (PDF), for ALF, HCS, ICF/IID and NF providers. This letter reminds providers that they must allow persons providing critical assistance and providers of essential services to enter the facility if they pass the facility’s COVID-19 screening.
September 5th, 2021
ICF/IID Leave During COVID-19 Rule Reinstated
HHSC has published IL 2021-42 ICF/IID Services During COVID-19 (PDF), which replaces IL 2020-43.
A resident must be discharged from the Intermediate Care Facility for Individuals with an Intellectual Disability or Related Conditions, with or without a contract to hold the resident’s placement in accordance with 26 TAC Section 261.227(j), if the resident is absent from the ICF/IID for one full day or more and the absence is not during leave described in 26 TAC Section 261.226.
August 1st, 2021
HHSC Publishes Updated COVID-19 Response Plan and Frequently Asked Questions for ICF Providers
HHSC Long-term Care Regulation updated the ICF COVID-19 Response Plan and FAQ document on July 29, 2021.
Read the updated ICF COVID-19 Response Plan (PDF).
Read the updated ICF FAQs (PDF).
March 24th, 2021
HHSC Adopts New Expansion of Reopening Visitation Emergency Rules for ICF Providers!!!
HHSC has adopted new Expansion of Reopening Visitation (PDF) emergency rules that establish criteria for expanded indoor and outdoor visitation as well as essential caregiver visits. These rules are effective March 24, 2021.
HHSC Publishes COVID-19 Response – Expansion of Reopening Visitation for ICF Providers (PL 2021-10)
HHSC has published Provider Letter 2021-10, COVID-19 Response – Expansion of Reopening Visitation (PDF) for ICF/IID providers. This letter replaces Provider Letter 2020-43. This letter describes the criteria for expanded indoor and outdoor visitation as well as essential caregiver visits.
March 22nd, 2021
ICF COVID-19 March 22 Webinar Recording Available
A recording of the March 22, 2021, ICF/IID COVID-19 Q&A with HHSC LTC Regulation and DSHS is available for those who could not attend.
Listen to the COVID-19 Q&A recording.
March 11th, 2021
Recording of ICF COVID-19 March 8 Provider Webinar Available
A recording of the March 8, 2021 ICF/IID COVID-19 Q&A with HHSC LTC Regulation and DSHS is available for those who could not attend.
Listen to the COVID-19 Q&A recording.
February 8th, 2021
Draft ICF/IID Rule Changes
- Draft ICF/IID Rule Changes- See link: https://documentcloud.adobe.com/link/track?uri=urn:aaid:scds:US:cf5ea154-04b4-4c75-8aec-7e230c3cfda0
- High Level Summary of Proposed Changes See link: https://documentcloud.adobe.com/link/track?uri=urn:aaid:scds:US:2aa689d6-3018-4ab9-8d75-04578f4c922d
February 7th, 2021
Feb. 8- ICF/IID Provider Bed Hold Payments Webinar &
Reminder to Provide Letter to Families
IL 2020-43, ICF/IID Services During COVID-19 directed program providers to give a copy of the letter attached to that IL to each resident who was:
- Absent from an ICF/IID
- Not on leave
- Was not discharged from the ICF/IID
If you have not provided the letter to residents who meet that criteria, do so immediately. It was to be complete by Oct. 5, 2020. You must assist residents in deciding to do only one of the following:
- Return to the facility.
- Continue to be absent from the facility, be discharged, but enter into an agreement with the ICF/IID to hold your place at the facility.
- Continue to be absent from the facility and be discharged.
HHSC Medicaid and CHIP Services will issue instructions to providers about entering COVID-19 therapeutic leave for days a resident was away from an ICF/IID to reduce the risk of COVID-19 transmission. Register for the following webinar to review the emergency rules authorizing this leave and the instructions for entering it.
ICF/IID Provider Bed Hold Payments Webinar
Friday, Feb. 5
9:30-11 a.m.
Register for the Bed Hold Payment Webinar.
The emergency rule authorizing payment for COVID-19 therapeutic leave (PDF) is effective Jan. 29, 2021.
January 19th, 2021
HHSC Updates the ICF COVID-19 Response Plan and FAQ Document
Please be sure to update your infection control and other related policies based on the updated Response Plan!!!
Let us know if we can help. We will be working on some of these addendums in the next few weeks.
HHSC Long-term Care Regulation updated the ICF COVID-19 Response Plan and FAQ document on Jan. 12, 2021.
Read the updated ICF COVID-19 Response Plan (PDF).
Read the updated ICF FAQs (PDF).
Jan. 25 ICF Provider COVID-19 Webinar with HHSC LTC Regulation
Long-term Care Regulation and the Department of State Health Services provide the latest information on the COVID-19 pandemic and take live questions from participants in this ICF provider webinar. Provider attendance is critical to staying current with COVID-19 requirements and guidance.
ICF/IID providers are strongly encouraged to attend this and all biweekly COVID-19 webinars with LTCR and DSHS.
Those using Internet Explorer may have difficulties registering for the webinar. Please try another browser such as Google Chrome or Microsoft Edge.
ICF Provider Webinar
Jan. 25, 2021
11 a.m. – 12:30 p.m.
Register for the COVID-19 Webinar.
Recording of ICF COVID-19 Jan. 11 Provider Webinar Available
A recording of the January 11, 2021 ICF/IID COVID-19 Q&A with HHSC LTC Regulation and DSHS is available for those who could not attend.
Listen to the COVID-19 Q&A recording.
January 10th, 2021
Informal Comments on Draft Rules for ICF/IID or Related Conditions
HHSC is accepting informal comments from stakeholders on the following draft rules, which are now posted on the HHS Rulemaking page. The comment period ends Jan. 19, 2021.
This project implements two bills from the 86th Legislature, Regular Session, 2019. House Bill (H.B.) 1848 contains required elements for infection prevention and control. H.B. 3803 limits the daily amount of an administrative penalty assessed against an ICF/IID. The project will also update rule references and agency names, amend rules to align with Centers for Medicare & Medicaid Services conditions of participation in the ICF/IID program, and edit the rules for clarity and consistency.
HHSC Publishes Revised Reporting Guidance for Long-Term Care Providers
(PL 20-37)
HHSC has published a revised version of Provider Letter 20-37, Reporting Guidance for Long-Term Care Providers (PDF). The revision includes information for ALF and ICF providers offering point-of-care testing for COVID-19 and clarifies reporting requirements for NF providers.
November 22nd, 2020
November 30 ICF Provider COVID-19 Webinar with HHSC LTC Regulation
LTCR and DSHS provide the latest information on the COVID-19 pandemic and take live questions from participants in this ICF provider webinar. Provider attendance is critical to staying current with COVID-19 requirements and guidance.
ICF/IID providers are strongly encouraged to attend this and all biweekly COVID-19 webinars with HHSC Long-term Care Regulation and the Department of State Health Services.
Those using Internet Explorer may have difficulties registering for the webinar. Please try another browser such as Google Chrome or Microsoft Edge.
ICF Provider Webinar
November 30, 2020
11 a.m. – 12:30 p.m.
Register for the COVID-19 webinar.
Recording of November 16 ICF COVID-19 Q&A Provider Webinar Available
A recording of the November 16, 2020 ICF/IID COVID-19 Q&A with HHSC LTC Regulation and DSHS is available for those who could not attend.
View the COVID-19 Q&A recording.
10/16/20
Important Information!
LTCR FORM 2195: Expansion of Reopening Visitation Status Attestation and Letter PL 20-43 (for ICF Only)
Note: to receive an approved general visitation designation, a small ICF/IID that cannot provide separate areas, units, wings, halls, or buildings for individuals who are COVID-19 positive, COVID-19 negative or unknown COVID-19 status, based on the status of the entire facility, must:
- have no facility-acquired COVID-19 cases in individuals for at least 14 consecutive days; and
- have no COVID-19 cases in staff.
An ICF/IID must provide instructional signage throughout the facility and proper visitor education regarding
- signs and symptoms of COVID-19;
- infection control precautions; and
- other applicable facility practices (e.g., the use of facemasks or other appropriate PPE, specified entries and exits, routes to designated visitation areas, and hand hygiene).
An ICF/IID that does not meet the criteria for a visitation designation must permit closed window visits and end-of-life visits for individuals regardless of their COVID-19 status, as well as essential caregiver visits for individuals with COVID-19 negative or unknown COVID-19 status.
Such an ICF/IID must also develop and implement a plan to meet the visitation criteria and submit the plan to the regional director in the Long-term Care Regulation (LTCR) region where the ICF/IID is located within five days of submitting the new 2195 Expansion of Reopening Visitation Status Attestation Form,
or
within five days of receiving notification from HHSC that the ICF/IID was not approved for general visitation designation.
LTCR Form 2195
Each ICF/IID must submit LTCR Form 2195 to the Regional Director in the LTCR region where the facility is located and must provide information about whether the ICF/IID meets or does not meet the criteria for expanded general visitation.
Each ICF/IID must submit a completed form 2195 to the Regional Director no later than October 31, 2020.
An ICF/IID that does not meet the visitation designation criteria must attest that it:
- is permitting closed window visits, end of life visits, and essential caregiver visits;
- will develop and implement a plan to meet the visitation designation criteria as defined in 26 TAC §551.47; and
- has included the plan with the form or will submit the plan within five business days of submitting the form.
To seek a designation for general visitation, an ICF/IID must complete LTCR Form 2195, Expansion of Reopening Visitation Status Attestation, to notify LTCR that the ICF/IID seeks a designation as a visitation facility.
The form must be emailed to the LTCR regional director in the LTCR region where the facility is located. Any applicable pictures and facility maps must also be included with LTCR Form 2195.
The LTCR regional director or designee will review the form within three working days of submission and notify the ICF/IID whether it has received been approved for a visitation designation.
An ICF/IID with previous approval for visitation does not have to submit LTCR Form 2195 or other documentation unless the previous visitation approval has been withdrawn, rescinded, or canceled, or was for only indoor or outdoor visitation instead of both indoor and outdoor visitation.
If approved, the ICF/IID must allow outdoor visits, indoor plexiglass visits, open window visits, and vehicle parades in accordance with the applicable emergency rule. HHSC LTCR can conduct an on-site visit to confirm an ICF/IID’s compliance with the requirements. If HHSC determines that the ICF/IID does not meet the requirements for the designation as a visitation facility, the ICF/IID must immediately stop all visitation except a closed window visit, end-of-life visit, and visits by persons providing critical assistance, including designated essential caregivers.
If, at any time after a visitation designation is approved by HHSC, the ICF/IID experiences an outbreak of COVID-19, the ICF/IID must notify the Regional Director in the LTCR Region where it is located that the ICF/IID no longer meets visitation criteria, and the ICF/IID must immediately stop all visitation, except a closed window visit, end-of-life visit, or visits by persons providing critical assistance, including essential caregivers.
The ICF/IID can submit a new request for designation when it meets all visitation criteria.
Under Section 37.10 of the Texas Penal Code, a person commits a criminal offense if he or she makes a false entry in a governmental record; makes, presents, or uses any record or document with knowledge of its falsity and intent that it be taken as a genuine governmental record; or makes, presents, or uses a governmental record with knowledge of its falsity. In addition, making a false statement on the attestation form can result in the imposition of an administrative penalty as described in Texas Health and Safety Code, Chapter 252, section 252.065(a)
Contact Information for Submitting LTCR Form 2195 to the LTCR Regional Director: https://hhs.texas.gov/about-hhs/find-us/long-term-care-regulatory-regional-contact-numbers
If you have any questions about this letter, please contact the Policy, Rules and Training Section by email at PolicyRulesTraining@hhsc.state.tx.us or call (512) 438-3161.
10/05/20
Deadline for Decision To Return to Facility/Payment to Hold Bed/Discharge Option
Information Letter No. 20-43 ICF/IID Services During COVID-19
10/03/20
Quick TIPS on New Changes for Expansion of Facility Visitation Rules & Other Policies Concerning COVID-19
Essential Caregiver Visits and Salon Services Visitor (Visitor types above and beyond normal designated visiting facility type)
Essential Caregivers are allowed even if the facility does not have designated visitation. They must be at least 18 years of age. Can be family, friend, guardian.
- 1.They must wear a facemask. 2. They must have evidence of negative COVID-19 test in the past 14 days and must be screened by facility staff prior to the visit. (Pay attention to the new additional symptoms and discontinuation of asking about international travel). 3. There is a 2- hr limit unless the facility allows a longer period of time. 4.They can have physical contact with their individual (and only their individual) 5.They must be limited to access to their person. 6.An assigned person must escort them to and from the location they are going to meet with the individual- room, outside… 7. The facility must approve the face mask they are going to wear or provide an appropriate mask. 8.If they do not have an appropriate face mask or the facility can’t provide one, they will need to reschedule the visit. 9. The facility must create an identifying badge for the essential caregiver or any salon service visitors. 10. The facility must have attestation stating the essential caregiver has visited and when they left (include time arrived, when they left, who they visited). 11. Essential caregivers must be trained on PPE and how to wear PPE, handwashing, and other infection control practices and there must be proof of this training that the facility maintains. 12..The individual must be COVID-19 negative to have an Essential caregiver visit or Salon Service Visitor.
- ***(If client status is unknown, the essential visitor would have to wear a face mask (not N-95, save that for your staff who need to work with COVID-19 + individuals), gown, gloves, goggles or face shield, so the facility might need to provide these other items)
Phase I Visitation Rules, no longer in effect!
Expanded Visitation Rules now in effect: The facility must apply with form 2194 for the Designated Visitation Facility, do not require all these same requirements an essential visitor (or Salon Service Visitors)- Designated visits include: open window visits, closed window visits, parade visits (open window or closed) outside visits, (and in plexiglass separate areas). Must use physical distance, visitor screening, individual will wear face mask or face covering if tolerated and the same for the visitor -they must wear a face mask or face covering. Provider Letter 20-38 has the link with form 2194 at the end of it. We encourage you to email the form. Most staff at HHSC are not in the office Visitation Designation department has 3 days to approve or deny the 2194 form request. Only the administrator or director can fill out form 2194!!
Previous Level 1 Attestation Approval: If you previously submitted the 2192 and received approval, you do not need to request a new one with form 2194, if your status is the same. If you want to change the visitation type, then you will need a new form. ( i.e. you did not want plexiglass partition previously and now you do.)
Small facilities must request visitation designation for the whole facility (unless you have a way to separate all cohort areas completely.) Do not fill out section #3. That is only for NF’s.
The provider must develop and must enforce policies and procedures including testing strategies for Essential Visitors, in addition to the testing that must occur at least 14 days prior to 1st visit and all other polices related, such as essential visitor requirements discussed above. Remember your testing strategies are required to provide expectations for the essential visitor for how often they will be required to test and when, for any visits they are making, after the initial “essential caregiver visit”.
For vehicle parades, the individual needs to remain 10 ft or more from the vehicle for safety.
Plexiglass booths on the inside must be approved by a life safety person for your region. Send in pictures. Does have to be a plexiglass barrier, does not have to be 2 and 3 sided.
**Closed window and End of Life visits are the only visits an individual may have if they are COVID-19 + (positive)
Remember staff should be assigned to an appropriate cohort (COVID-19 +, COVID-19 Negative or Unknown) then they should stay with that Cohort. In addition, there should be a policy at the facility for limiting the sharing of staff. If you need help with your policy concerning “COHORTS” and ” Mitigation of COVID-19 by Limiting Sharing of Staff”, please contact us. I do have some policies you can purchase if needed. Please contact me at: info@twogetherconsulting.com
FAQ’s (June 2020)
Answer: Regulations regarding ICF/IID discharge have not been waived. A discharge, even on a temporary emergency basis, requires that key developmental, behavioral, social, health and nutritional information be shared with the accepting facility in the community or non-facility provider. CMS is aware that staffing shortages and/or client surges due to the PHE create a high demand on available staff time that makes it difficult to complete a full discharge summary for each client. Each ICF will need to evaluate what amount and detail of documentation is necessary to ensure that critical health information is shared with the accepting facility or other provider. When available and if appropriate, the Interdisciplinary Team (IDT) should maximize the use of telehealth for the completion of a client’s discharge plan during the PHE.
Answer: Yes. CMS has temporarily waived certain regulatory requirements providing flexibilities to assist RHCs and FQHCs in furnishing services during the COVID-19 PHE. This includes temporarily modifying the following:(a)50% mid-level staffing requirement for RHCs;(b)Physician supervision requirement for nurse practitioners (NPs), to the extent permitted by State law; and(c)Location requirements for existing RHCs and FQHC to allow additions of temporary service locations.Please see https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf for additional waiver information. Additional flexibilities, including guidance for RHCs and FQHCs furnishing telehealth services during the PHE, are also described in this CMS MLN Article: https://www.cms.gov/files/document/se20016.pdf
Answer: Yes. During the COVID-19 PHE, CMS is allowing currently approved RHCs/FQHCs to provide patient care services in temporary expansion sites to help address the urgent need for supplementary care. These temporary sites are not restricted to the rural/shortage area location requirements. Each location is obligated to follow RHC/FQHC regulations to the extent not waived. Therefore, the RHC/FQHC may provide services provided at a temporary location under the CMS Certification Number (CCN) for the permanent location. The RHC/FQHC is expected to be operating in a manner not inconsistent with its state’s emergency preparedness plan. Note: FQHCs must also have an updated Health Resource and Service Administration (HRSA) Notice of Award, expanding the scope of service to include the temporary location(s) to support response to the COVID-19 PHE.
My RHC participates in Medicare through one of the two CMS-approved RHC Accreditation Organizations (AOs). Do waivers of CMS regulations apply to CMS-approved accrediting programs? Do I need to notify the AO of my desire to temporarily add a service location during the COVID-19 PHE?
Answer: To assist RHCs and FQHCs in furnishing service during the COVID-19 PHE, CMS has provided additional flexibilities related to billing for services. These temporary flexibilities currently include Expansion of Virtual Communication Services for RHCs and FQHCs to include online digital evaluation and management services using patient portals, and Revision of Home Health Agency Shortage Area Requirement for Visiting Nursing Services Furnished by RHCs and FQHCs. Please see the Medicare FFS Billing FAQ document available at https://www.cms.gov/files/document/03092020-covid-19-faqs-508.pdf. Please see Section II.L of the Interim Final Rule with Comment Period, “Medicare and Expanded Flexibilities for Rural Health Clinics (RHCs) Medicaid Programs; Policy and Federally Qualified Health Centers (FQHCs) During Regulatory Revisions in Response to the COVID-–19 Public Health Emergency (PHE)”(85 FR 19230, 19253), available at https://www.federalregister.gov/documents/2020/04/06/2020-06990/medicare-and-medicaid-programs-policy-and-regulatory-revisions-in-response-to-the-covid-19-public, for more information on regulatory changes for RHCs and FQHCs.
Webinar info: How to prevent infectious diseases and the spread of infectious diseases in ICF-Specifically COVID-19 (May 27th, 2020)
See the following pdf link from HHSC
https://hhs.texas.gov/sites/default/files/documents/doing-business-with-hhs/providers/long-term-care/icfiid-covid-updates-qa-webinar-may-27-2020.pdf
 Survey Operations Resume as of June 15th, 2020
Survey Operations Resume as of June 15th, 2020
 Providers Must Log Residents’ Leave
Providers Must Log Residents’ Leave
All community-based ICF/IID program providers must submit an absence request in the TMHP ICF/IID Online Portal for a resident away from the facility for one or more full days. See the TMHP ICF/IID Online Portal User Guide (PDF) starting on page 52 for more information. The program provider must submit a return request within three days after a resident returns to the facility. See page 58 of the Online Portal User Guide for an explanation.
Please ensure that information entered in the portal for absences is current. HHSC uses the information to determine how many residents are absent from their facilities and the length of those absences.
PolicyRulesTraining@hhsc.state.tx.us or call (512) 438-3161.
Provider Joint Training Opportunities
Check out Joint Provider Trainings link from HHSC, for more info:
https://apps.hhs.texas.gov/providers/training/jointtraining.cfm#course_112
Survey Process for ICF Has Changed: Please Be Aware
You may check your SOMA (Surveyor’s Operational Manual for ICF in Appendix J) for these changes.
https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/som107ap_j_intermcare.pdf
http://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/2018Downloads/R178SOMA.pdf
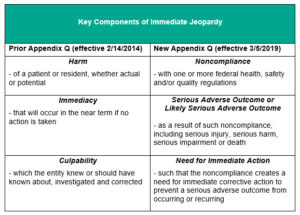
Appendix Q of SOMA (Immediate Jeopardy) Changes
CMS clarifications letter
SOMA appendix Q Section Immediate Jeopardy
https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/som107ap_q_immedjeopardy.pdf
|
LON & Facility Size |
Current Rates |
Proposed Rates |
|
SMALL |
|
|
|
LON 1 |
$144.25 |
$150.31 |
|
LON 5 |
$160.74 |
$167.90 |
|
LON 8 |
$182.82 |
$191.85 |
|
LON 6 |
$223.88 |
$236.59 |
|
LON 9 |
$406.11 |
N/C |
|
MEDIUM |
|
|
|
LON 1 |
$118.04 |
$123.14 |
|
LON 5 |
$134.06 |
$140.24 |
|
LON 8 |
$158.90 |
$166.92 |
|
LON 6 |
$190.24 |
$200.79 |
|
LON 9 |
$385.84 |
N/C |
|
LARGE |
|
|
|
LON 1 |
$112.09 |
$116.30 |
|
LON 5 |
$119.64 |
$124.64 |
|
LON 8 |
$133.22 |
$139.44 |
|
LON 6 |
$179.40 |
$188.96 |
|
LON 9 |
$387.25 |
N/C |